Membrane electrolysis sodium hypochlorite jenareta muchina wakakodzera mvura yekunwa disinfection, kurapa kwemvura yetsvina, hutsanana uye kudzivirira denda, uye kugadzirwa kwemaindasitiri, inogadzirwa neYantai Jietong Water Treatment Technology Co., Ltd., China Water Resources uye Hydropower Research Institute, Qingdao University, Yantai University uye mamwe masangano ekutsvagisa nemayunivhesiti. Imhando yemuchina wekugadzira yakakwira-concentration sodium hypochlorite mhinduro panzvimbo, inogutsa zvakanyanya kudiwa kweyakakwira concentration sodium hypochlorite zvigadzirwa, uye inogadzirisa matambudziko ekufambisa nekuchengetedza. Membrane sodium hypochlorite jenareta inogadzirwa naYantai Jietong Water Treatment Technology Co., Ltd. ndiyo yega tekinoroji kambani muChina inogona kugadzira yakakwira concentration sodium hypochlorite zvigadzirwa panzvimbo. Membrane electrolysis brine sodium hypochlorite jenareta inogona kuburitsa 4-12% yakakwira concentration sodium hypochlorite mhinduro ine yakavharwa loop yedosing uye kugadzira yakazara otomatiki otomatiki.
Izvi zvinotevera Working Theory
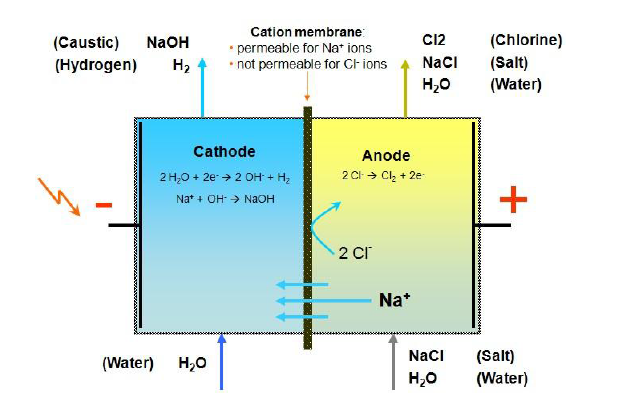
Musimboti wekutanga we electrolytic reaction ye membrane electrolysis cell ndeyekushandura simba remagetsi kuita kemikari simba uye electrolyze brine kugadzira NaOH, Cl2 neH2 sezvaratidzwa pamufananidzo uri pamusoro. Mukamuri yeanode yesero (kurudyi rwemufananidzo), iyo brine inoiswa muNa + uye Cl- muchitokisi, umo Na + inotamira kukamuri ye cathode (kuruboshwe rwemufananidzo) kuburikidza neanosarudza ionic membrane pasi pechiito chekuchaja. Iyo yakaderera Cl- inogadzira chlorine gasi pasi peanodic electrolysis. Iyo H2O ionization mukamuri yecathode inova H + uye OH-, umo OH- inovharwa neanosarudza cation membrane mukamuri yecathode uye Na + kubva mukamuri yeanode inosanganiswa kugadzira chigadzirwa NaOH, uye H + inogadzira hydrogen pasi pecathodic electrolysis.


Nguva yekutumira: May-31-2024

